Drug-Drug Interaction Checker API & Plugin
Prescribing just got safer
Improve patient outcomes, assist in clinical decision-making, and reduce medical errors.

Drug-Drug Interaction Checker API & Plugin
Improve patient outcomes, assist in clinical decision-making, and reduce medical errors.

Search by ingredient identifiers, product identifiers (such as NDC codes), RxNorm codes, or product concept identifiers.
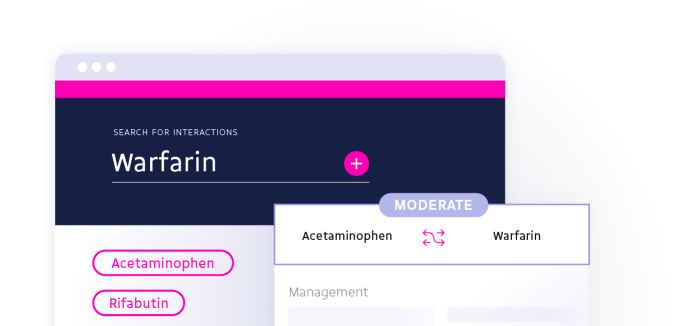

https://api.drugbank.com/v1/ddi?drugbank_id=DB00316,DB00682{
"interactions": [
{
"ingredient": {
"drugbank_id": "DB00316",
"name": "Acetaminophen"
},
"affected_ingredient": {
"drugbank_id": "DB00682",
"name": "Warfarin"
},
"description": "The risk or severity of adverse effects can be increased when Acetaminophen is combined with Warfarin.",
"extended_description": "Chronic oral acetaminophen use at a dose of 4,000 mg/day has been demonstrated to cause an increase in the international normalized ratio (INR) for some patients who have been stabilized on sodium warfarin as an anticoagulant. This interaction is thought to occur through augmented effects on vitamin K antagonism, increasing the risk of bleeding.",
"action": "increase_adverse_effects",
"severity": "moderate",
"subject_dosage": null,
"affected_dosage": null,
"evidence_level": "level_1",
"management": "Use this combination with caution. Avoid warfarin doses of 4, 000 mg/day or higher when acetaminophen is used, and regularly monitor INR. Adjust drug dosage as necessary, according to clinical response."
}
],
"total_results": 1
}Choose up to 40 different drugs and check for all interactions between them in a single request.
A brief description of the drug-drug interaction.
A detailed description of why the interaction occurs and the effect of the interaction.
The severity of each drug interaction is rated as minor, moderate or major.
A rating for the strength of the evidence supporting each drug interaction.
The recommended procedure to deal with this type of drug-drug interaction.
Get the full value of our Clinical API without the costly development hours.
Our meticulously designed, easy-to-use plugin can be quickly integrated into your systems.
Contact our sales team to add
Drug-Drug Interaction Checker to your software.